Why Should “Zero Defects” Be Your Core Manufacturing Goal?
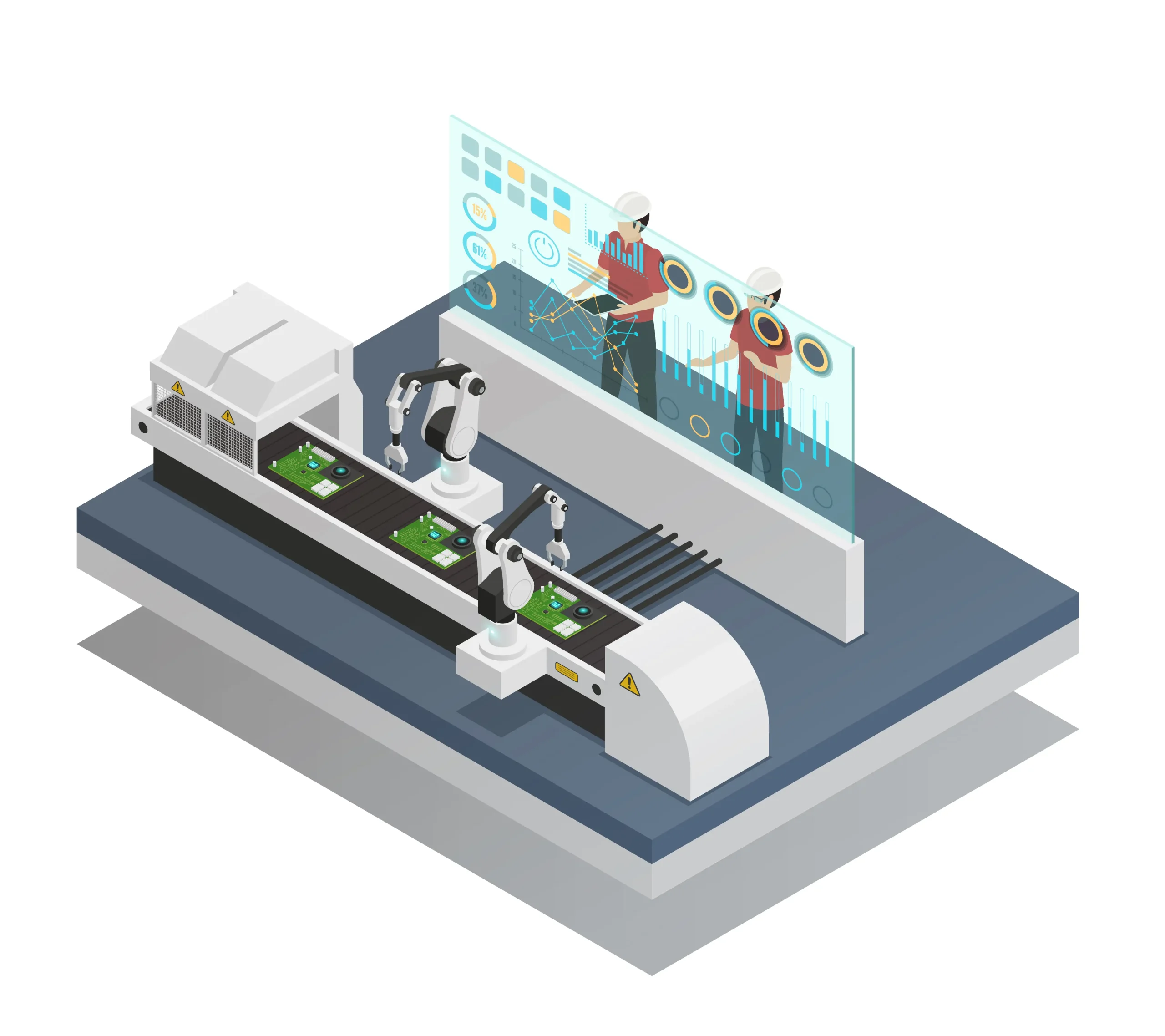
In aerosol production, a zero defect rate strategy is the cornerstone of operational sustainability. Even a single faulty unit can lead to product recalls or safety hazards. Automated quality assurance systems minimize human error, protecting the reputation of your production line and ensuring maximum consumer safety. Achieving this goal is the most significant power that distinguishes your brand from global competitors.
Is Liquid Dose Control Truly the First Critical Step in Production?
A correct manufacturing process begins at the foundation. Liquid dose control ensures production optimization by rejecting incorrect weights before they reach the gas filling stage. The precision provided at this first stage directly affects the success of the entire production line. Eliminating faulty liquid quantities prevents the waste of expensive propellants and packaging components. The precision provided at this first stage directly affects the success of the entire production line. For those operating in highly regulated sectors, understanding the liquid filling validation in pharmaceutical production is a vital next step to ensure these precision standards meet global healthcare requirements.
How Risky is Skipping Valve Placement Inspection Before Gas Filling?
Skipping valve placement inspection creates an unpredictable risk factor on the production line. Missing, tilted, or incorrectly seated valves must be removed from the line immediately. Such faulty valves are dangerous for gas filling; high-pressure filling leads to uncontrolled leaks, mechanical damage, and serious safety violations. This critical sorting process performed right before the gas stage is one of the fundamental safeguards of operational security.
Why is High-Accuracy Gas Weight Measurement the Third and Final Control Layer?
Propellant weighing is the final and most critical seal of product performance and safety. High-precision weighing systems detect even micro-deviations, ensuring that every aerosol complies 100% with internal pressure and filling standards. This stage, representing the most vital aerosol filling strategies, validates the shelf life and user safety of the final product.
How Can This Comprehensive Control Structure Succeed Across All Technologies (BOV and Conventional)?
Sora Machine brings Bag-on-Valve (BOV) and Conventional filling technologies together under the same high-engineering roof. Leveraging the multi-layer quality control power, it offers modular solutions that guarantee product sterility and bag integrity in BOV systems, while combining high production speeds with formulation accuracy in conventional lines. This inspection architecture, which can be integrated into both production types, carries the facility to global standards with a zero-defect principle, regardless of the method.