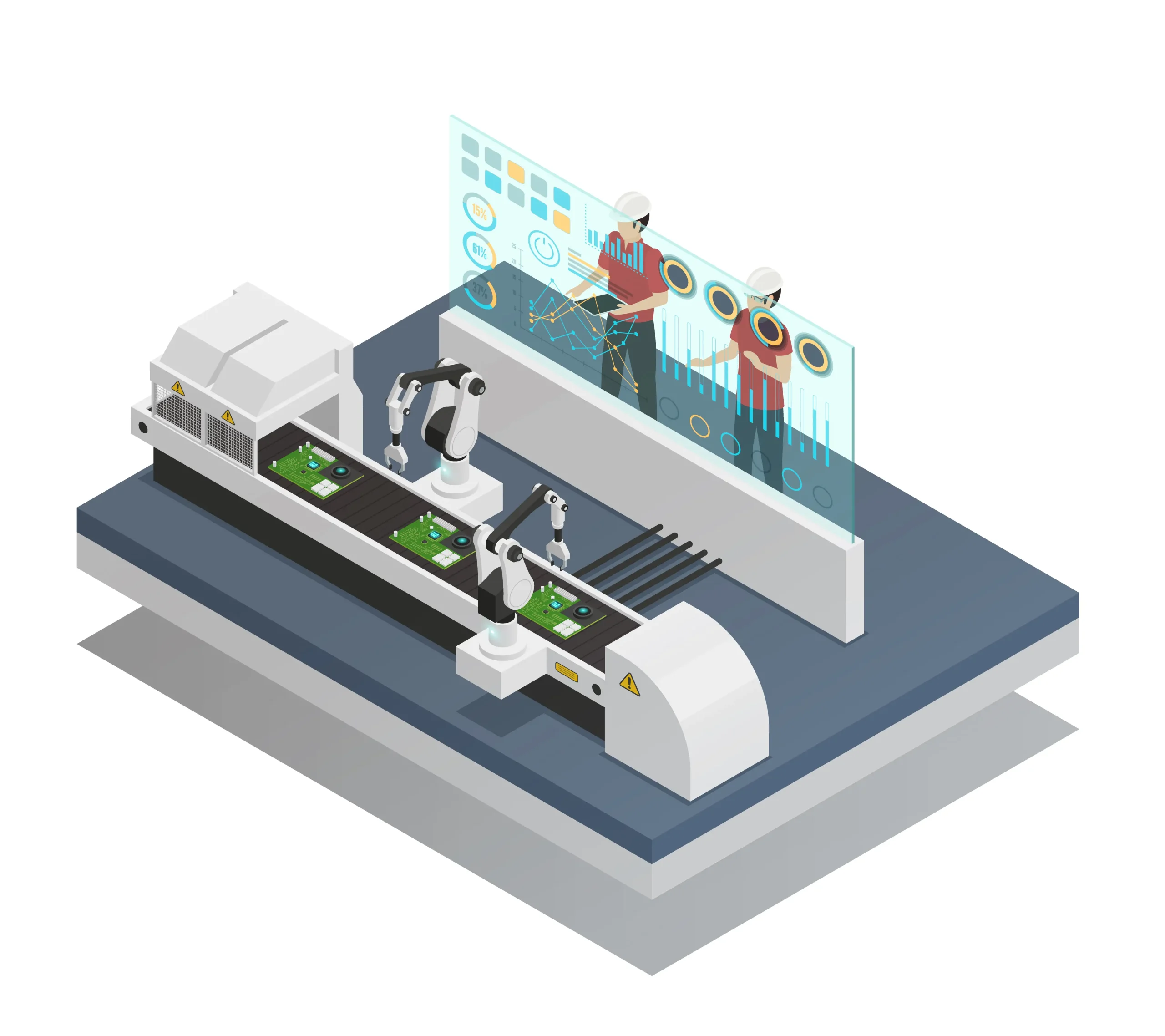
Why are Product Loss and Regulation Stress the Biggest Challenges in Vial Filling?
The greatest challenges encountered in pharmaceutical vial filling are the preservation of sterile areas, the requirements for high-precision dosing, and the pressure to comply with strict cGMPCurrent Good Manufacturing Practices: Regulations enforced by regulatory bodies like the FDA to ensure pharmaceutical quality. (current good manufacturing practices) standards. These challenges lead to significant product loss and high regulatory stress, especially in the production of expensive drugs (biologics, vaccines).
What are the Main Hazards Leading to Pharmaceutical Product Loss?
Vial filling occurs in the sterile filling zone, which is described as a critical “battleground,” where even the slightest microscopic contamination can jeopardize an entire drug batch. The fundamental hazards that cause product loss and rejection are:
Contamination
Risk: The risk of contamination is ever-present in the aseptic filling process, which is critical for the product’s safety and efficacy. Contamination can stem from particulate matter, operator-sourced human errors, or environmental fluctuations.
Lack of Dosing Precision
Accurate dosing is vital, particularly for valuable injectable drugs such as vaccines, biologics, and small-molecule drugs. A very low margin of error is expected (e.g., some filling systems aim for 0.5 precision). Incorrect filling results in product rejection. To prevent these financial losses, implementing applicable solutions to reduce waste in liquid filling lines is essential for maintaining a sustainable production cycle.
Inadequate Environmental Control
Maintaining ultra-low particle counts and precise temperature and humidity management has a direct impact on product integrity.
Equipment Failure and Downtime
Failures in traditional filling lines lead to prolonged downtimes, consequently causing expensive products to remain on the line and be lost.
How to Manage High Regulatory Stress and cGMP Standard Compliance?
The pharmaceutical industry is strictly regulated by stringent regulatory frameworks such as cGMP (Good Manufacturing Practices) and 21 CFR Part 11. Factors increasing regulatory stress and the areas where they must be mitigated include:
Data Integrity
The consistency and reliability of processes are mandatory. The adoption of robotic systems and automation helps meet these requirements by offering consistent and reliable processes.
Aseptic Area Management
Human intervention must be minimized by using isolator technology and RABSRestricted Access Barrier Systems: Enclosures that provide a physical barrier between operators and the sterile process to reduce contamination. (Restricted Access Barrier Systems) beyond traditional cleanrooms. This facilitates compliance with new standards like EU GMP Annex 1.
Validation Challenges
Since specialized filling systems are required for new or personalized treatments (biologics, small-volume, high-mix productions), the fact that traditional lines can take two to three years from design to full qualification prolongs the time-to-market.
How Do the Global Top 10 Manufacturers Address These Issues, and How Do Superior Technologies Prevent Product Loss?
Global market leaders, such as Sora Machine, are increasing product precision and thus product yield by utilizing robotics and artificial intelligence (AI) integration, modular systems, and in-process control (IPCIn-Process Control: Checks performed during production to monitor and, if necessary, adjust the process to ensure final product quality.) mechanisms to minimize contamination risk.
How Do Artificial Intelligence (AI) and Robotics Integration Reduce Product Loss in Vial Filling?
The robotic vial filling machine market is a dynamic sector fueled by the need for automation, projected to reach $867.56 million USD by 2034. Artificial Intelligence (AI) is transforming this market:
Increased Precision and Speed
Robotic arms and integrated vision systems reduce human intervention, facilitating precise handling and filling, which lowers contamination risks.
Real-Time Process Monitoring
AI-supported systems can monitor processes in real time, predict potential equipment failures in advance, and optimize performance, thereby reducing downtime.
Advanced Quality Control
AI-enabled machine vision systems can detect defects such as cracks and contamination in vials with higher accuracy and speed than human operators.
Flexibility
These systems automatically adapt to different vial sizes, product viscosities, and filling requirements, shortening changeover times.
How to Ensure High Precision and Product Integrity in Aseptic Filling?
High-precision technologies are critical for minimizing the loss of valuable drugs. These technologies include:
100% IPC (In-Process Control)
Advanced filling systems have the capability to perform one-hundred percent weight checks of all filled vials. This ensures that every single vial receives the correct dose, significantly reducing product loss and waste.
Optimized Design for Low Product Loss
Some filling systems feature compact designs that allow product tanks to be positioned close to the machine, minimizing product loss in transfer piping.
Dual Filling Capability
Certain modular systems can perform both powder and liquid filling within a single isolator chamber, enabling the processing of complex formulations (e.g., liquid-powder combinations or a second filling after lyophilization) on a single line.
How Does Sora Machine Enhance Process Efficiency and Regulatory Security in Vial Filling?
Considering the industry challenges, it can be assumed that high-performance Sora Machine solutions minimize potential problems by using industry-leading features such as modularity, high-volume production capacity, and 100% weight checking for aseptic operations.
How Do Sora Machine Vial Filling Lines Minimize the Risk of Contamination?
Sora Machine uses fully integrated isolator technology to minimize contamination risk. These systems completely separate human intervention from the critical filling area (aseptic environment) and use sterilization technologies like VHP (Vaporized Hydrogen Peroxide) cycles to ensure the product remains sterile.
What Precision Controls Does Sora Machine Equipment Offer to Prevent Product Loss in Expensive Drugs?
Sora Machine filling solutions integrate In-Process Control (IPC) weighing systems, allowing each vial to be automatically checked after filling. This high-precision dosage control prevents the loss of valuable products due to dosage errors.
Can the Sora Machine Vial Filling Machine Fill Both Liquid and Powder Products?
Sora Machine’s advanced modular filling platforms feature flexible configurations that allow alternative use of both liquid/suspension and powder filling systems within a single machine frame. This modularity is ideal, especially for high-mix, low-volume production requirements.
What Level of Automation Does Sora Machine Offer for Compliance with New Regulations?
Sora Machine maximizes compliance by offering full automation with robotic and servo-controlled systems. This reduces human error and ensures processes are consistent and repeatable, thereby easing audit stress.
How Fast Can Sora Machine Vial Filling Lines Operate?
In response to the market’s demand for speed, Sora Machine offers high-performance machine series capable of production. This speed is critical for meeting the growing demand for injectable drugs.